Burst spinal cord stimulation in a patient with a cardiac pacemaker
Fattibilità dell’impianto di SCS con stimolazione Burst
in un paziente con pacemaker cardiaco
Casi clinici
Pathos 2016; 23; 2. Online 2016, Sep 9
_________________________________________
Giuliano De Carolis,* Giulio Zucchelli **
Head Physician, Anesthesiology and Pain Therapy Unit
Azienda Ospedaliero Universitaria Pisana, Pisa *
Cardiac Thoracic and Vascular Department
Azienda Ospedaliero Universitaria Pisana, Pisa **
_______________________________________________
Summary
A 74-year-old male suffering from neuropathic pain in the distal portion of the lower limbs had obtained no relief from medical treatments. Additional comorbidities included pacemaker-dependent complete (3rd degree) atrio-ventricular block and a prolactin-secreting adenoma of the pituitary gland. An electrocatheter was implanted in the epidural space, with the active leads spanning the T10-T11 vertebral levels. Intraoperative stimulation tests were performed in both tonic and burst modes while pacemaker activity was monitored in real time. SCS stimulation intensity was increased in steps up to the threshold of patient tolerability (7.5 mA tonic, 3.9 mA burst). Pacemaker sensitivity was set to the maximum (0.5 mV): during the procedure, no interference with pacemaker sensing was observed. To the authors' knowledge, this is the first case report on the feasibility of burst SCS in a pacemaker-dependent patient. The report suggests that burst stimulation is safe in such patients.
Riassunto Un paziente maschio di 74 anni si presentava alla nostra osservazione con dolore neuropatico nella porzione distale degli arti inferiori non responsivo ai precedenti trattamenti medici. Si rilevavano inoltre comorbidità con blocco atrio-ventricolare pacemaker-dipendente (terzo grado) e adenoma prolattina secernente della ghiandola pituitaria. Per trattare il dolore neuropatico è stato inserito un elettrocatetere nello spazio epidurale a livello T10-T11. Durante l’operazione i test di stimolazione sono stati eseguiti sia in Tonico che in Burst monitorando l’attività dell’pacemaker in tempo reale. L’intensità di stimolazione SCS è stata aumentata progressivamente fino alla soglia di tollerabilità del paziente (7,5 mA tonico, 3,9 mA burst). La sensibilità del pacemaker era impostata al massimo (0,5 mV). Durante la procedura, non è stata osservata alcuna interferenza con rilevamento pacemaker. I risultati del caso clinico sono in accordo con la letteratura, che evidenza la sicurezza dell’impianto di neurostimolatore con stimolazione tonica in pazienti portatori di pacemaker cardiaco. Questo caso utilizza la stimolazione Burst, che a pari della tonica si è dimostrata sicura in pazienti con pacemaker cardiaco.
Key words Burst spinal cord stimulation, cardiac pacemaker, chronic neuropathic pain, safety, bradiarrhythmias
Parole chiave Stimolazione del midollo spinale, pacemaker cardiaco, dolore neuropatico cronico, sicurezza, bradiaritmie
Introduction
Background: Spinal cord stimulation (SCS) is a common treatment for medically intractable neuropathic pain.1,2 Recently, burst stimulation has been introduced as a new stimulation method to provide pain relief without generating paresthesia.3-5
Pacemaker-dependent cardiac conditions are considered a relative contraindication for SCS;6-10 indeed, most contemporary pacemakers operate in the demand mode: as they monitor cardiac activity, they may be inhibited by extracardiac electrical activity, such as the electrical field generated by SCS devices.
Materials and methods
A 74-year-old man presented with chronic neuropathic pain in the distal portion of the lower limbs. Previous medical treatments (pregabalin, oxycodone, medical cannabis) had not provided noticeable relief. He described his pain as a continuous burning sensation extending from the soles of the feet up to the ankles, more severe on the left side, and with intense allodynia when wearing socks. The pain was so intense as to impair deambulation. Additional comorbidities included a complete (3rd degree) atrio-ventricular block, which was treated with a cardiac pacemaker 6,7 (Zephyr DR 5826, St . Jude) set to DDDR pacing at 80 bpm. The pacemaker generator was embedded in a subcutaneous pocket in the upper left region of the thorax. The patient also suffered from a prolactin-secreting adenoma of the pituitary gland.
Before implantation of the electrocatheter for SCS, a CT scan and an EMG were performed to confirm the diagnosis of peripheral neuropathy.
After the risks and benefits of SCS had been explained to the patient, he consented to try SCS . Under x-ray guidance, one Octrode lead was inserted by means of a peridural approach, with the tip reaching the T10-T11 level. Correct electrode placement was tested by generating paresthesia in the areas in which the patient reported pain. In collaboration with cardiologists from the specialized pacemaker unit, we tested for potential interference between SCS and the cardiac pacemaker by temporarily setting SCS and the pacemaker in such a way as to maximize the probability of interference. Specifically, the pacemaker sensitivity threshold was reduced to the most sensitive value (0.5 mV) while pacemaker activity was fully monitored. SCS stimulation was tested in both tonic and burst modes by increasing the stimulation intensity stepwise up to the maximally tolerated energy output. Moreover, stimulation was performed via the leads nearest to the heart (lead 1 and 2). In the tonic mode, stimulation with a frequency of 80 and current of 7.5 mA was reached; in the burst mode, stimulation with 40 Hz frequency, 500 Hz intra-burst frequency and a maximal current of 3.9 mA was reached (clinical target: 0.45 mA). As seen in Figures 1-2, no sensing activity was evident on pacemaker monitoring in both tests. After the tests, both the pacemaker and SCS were reset to clinically chosen parameters. After two weeks of burst stimulation, the patient reported significant pain relief (70%) and opted to undergo permanent implantation.
Discussion
Implantation of cardiac pacemakers is an effective and common treatment for bradiarrhythmias. The presence of a cardiac pacemaker has been considered a relative contraindication for SCS, owing to possible inter-device interference.8-12
The risk of the SCS signal being interpreted as cardiac activity by the pacemaker depends on the sensing threshold and the sensing mode (bipolar or unipolar) of the pacemaker. Moreover, the SCS parameters are also important, with the risk of interference depending on the output mode (bipolar o unipolar), frequency and energy of the stimulation. In this case report, both the pacemaker and the SCS generator were configured in the bipolar mode and all the other parameters were tested at maximum values. This experience suggests that SCS in the burst mode and with bipolar output is not necessarily contraindicated in patients with bipolar pacemakers. However, since interference may cause serious cardiac events, it is important to perform tests in order to ensure the absence of pacemaker sensing during SCS.
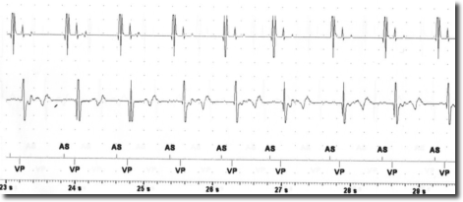
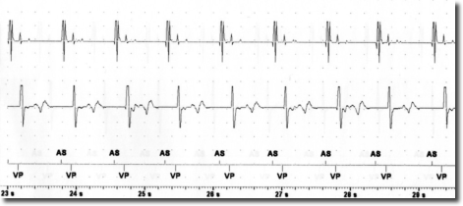
Conclusion
We suggest that it is safe to implant a spinal cord stimulator using burst stimulation simultaneously with a pacemaker provided adequate precautions are taken to prevent interdevice interference.
Conflict of interest
I certify the study has not been sponsored. I certify there are no potential conflicts of interest or financial conflicts, within the past 5 years and foreseeable future.
Published
9th Septemper, 2016
Correspondence
Giuliano De Carolis
giulianodecarolis@gmail.com
References
6) Bohm RP Jr et al. Treatment of complete atrio-ventricular block in a patas monkey using a permanent cardiac pacemaker. Lab Anim Sci 1991;
7) Ihenacho HN et al. Atrio-ventricular block (heart block) treated by cardiac pacemaker. Niger Med J; 1979.